|
|
About beta turn types
A beta-turn is a region of the protein involving four consecutive residues where the polypeptide chain folds back on itself by nearly 180 degrees (Lewis et al. 1971, 1973; Kuntz 1972; Crawford et al. 1973; Chou and Fasman 1974). It is these chain reversals which give a protein its globularity rather than linearity.
The ß-turn was originally identified, in model building studies, by Venkatachalam (1968). He proposed three distinct conformations based on phi,psi values (designated I,II and III) along with their related turns (mirror images)which have the phi, psi signs reversed (I',II' and III'), each of which could form a hydrogen bond between the main chain C=O(i) and the N-H(i+3). Subsequently, Lewis et al. (1973) examined the growing number ofthree-dimensional protein structures and suggested a more general definition of a ß-turn. This stated that the distance between the Calpha(i) and the Calpha(i+3) was < 7Å and the residues involved were not helical. They found that 25% of their extended ß-turns did not possess the intraturn hydrogen bond suggested by Venkatachalam. To
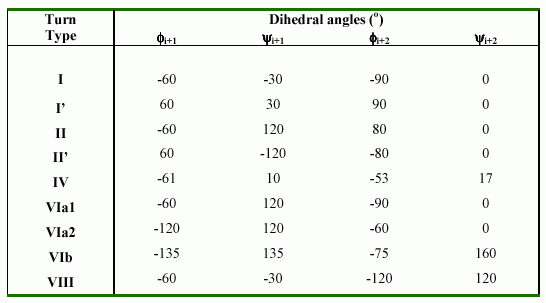
include the new data they extended the classification of ß-turns to 10 distinct types (I,I',II,II',III,III',IV,V,VI and VII). These classes were defined not only by phi,psi angles, but also less stringent criteria. Hutchinson and Thornton (1994) has since reappraised the situation, and has suggested that there are 9 distinct types (I,I',II,II',IV, VIa1, VIa2, VIb and VIII) based on
phi,psi ranges, along with a miscellaneous category IV. In present study, we have used this classification. The following table shows the nine beta-turn types with their dihedral angles:
The following image shows the two most common type of beta-turns: Type I and II.
|





